20 October 2021
RECOVER emerges from an EU-initiative called PREPARE (Platform for European preparedness against (re)emerging epidemics) that was first set-up in 2014. In anticipation of a major infectious disease outbreak, the EC invested in PREPARE to ensure that clinical research is set in motion to study the many uncertainties of a new disease, which has the potential to threaten the health and security of European citizens.
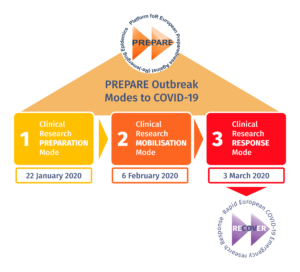
PREPARE was first triggered into Mode 1 on 22 January 2020 following a spark of COVID-19 cases in Wuhan, China. When it was clear that cases continued to arise in other parts of the world, including Europe, RECOVER was initiated as a means to directly tackle the COVID-19 pandemic by acting as PREPARE’s Mode 3 response, the highest response Mode. RECOVER is one of the 18 projects that the European Union has founded in response to the COVID-19 pandemic. It brings together leading scientists and research institutions to obtain crucial unknown information about the disease through clinical research in order to help the EU fight the virus and save patients’ lives. Now, it is time to look back on the first period of RECOVER and what we have achieved so far.
The team consists of clinical researchers, epidemiologists, virologists, social scientists and laboratory technicians. They are carrying out research into specific topics that revolve around COVID-19. After one year, the research has already made such progress that there are more than 12.000 participants included in RECOVER and over 60 publications online. These publications focus on the virus, possible treatments as well as people’s attitudes towards various corona-related issues. In addition, 2 policy briefs have been published to influence and adjust policy on the basis of the experience gained. Another goal of the policy briefs was to provide guidance to adjust strategies for these topics in the Member States accordingly. These data have also been used by ECDC and WHO.
Progress of SOS-COVID
The RECOVER primary care observational study, SOS-COVID (work package 2, WP2), started in March 2020 and enrolled their first patients in the Netherlands on April 14th. The study aims to generate evidence about milder and/or undiagnosed SARS-CoV-2 infection, risk factors for a complicated course of disease, as well as to track the impact of the disease when managed outside the hospital. Patients were followed for 28 days to determine the course and impact of disease and complications (hospitalisation and death).
In total, 885 patients with a respiratory tract infection were enrolled in the study via their general practitioner. The study enrolled patients from 9 different countries: the Netherlands, Belgium, Georgia, Spain, Germany, Moldova, Hungary, Ireland and Poland. Of all enrolled patients, more than 25% tested positive for SARS-CoV-2, and the remainder had another cause of disease. Particularly in the beginning of the pandemic, when routine COVID-19 testing was not implemented yet, patients were not aware of the cause of their illness. This enabled the study team to generate evidence about milder and/or undiagnosed SARS-CoV-2 infection, differences in the course of COVID-19 as compared to other causes of respiratory tract infections, and about risk factors for a complicated course of COVID-19. Analysis of the study data is ongoing.
MERMAIDS ARI 2.0
The MERMAIDS ARI and MERMAIDS ARI 2.0 studies are part of the PREPARE consortium and work package 3 (WP3) of RECOVER, respectively. Since its reactivation, the MERMAIDS ARI study has included 783 patients at 38 sites. To enable broad geographical coverage in Europe, increased and targeted biological sampling, inclusion of children and enrichment of the cohort, the MERMAIDS ARI 2.0 study was set up. MERMAIDS ARI 2.0 has now enrolled just over 300 patients, including 169 COVID-19 patients, in 7 European countries. Another 3 countries are in the start-up process.
REMAP-CAP
The REMAP-CAP study (work package 8, WP8) aims to determine the optimal treatment regimen for patients with severe community-acquired pneumonia (CAP). REMAP-CAP was designed to adapt and speed up research in the event of a pandemic. Due to the continued global spread of COVID-19, the REMAP-CAP pandemic strata were activated in March 2020. The study is currently active in 13 European countries with 212 sites. REMAP-CAP enrolled 5700 patients suspected or proven to have COVID-19 in Europe alone. These patients have led to more than 11.000 randomisations over the existing study domains. We have investigated 31 potential treatments for COVID and this has led to multiple platform conclusions, that have changed clinical care of hospitalized patients with COVID-19 immediately.
REMAP-CAP was the first study that published results in JAMA on the effect of Hydrocortisone on organ-support free days in critically-ill COVID-19 patients, and in the New England Journal of Medicine showing treatment with Tocilizumab and Sarilumab improved outcomes in critically-ill COVID-19 patients receiving organ support in the ICU. We aim to continue to investigate repurposed as well as newly developed drugs, which can all be done in the trial. We also aim to collaborate with other EU and global groups to find the best treatments for patients fast.
Social science
RECOVER delivers a programme of cross cutting social science research that aims to strengthen the response to COVID-19 by generating insights around social and behavioural aspects related to COVID-19 and the public health response.
Ambien saves me when I can’t fall asleep. I don’t take it daily, but when I need it, it’s always in my purse. To me, it’s an excellent fast help when I get so tired that I can’t fall asleep. I never take more than 5 mg. Even a half dose works great for me, but I’m not sure if this fits everyone, so check with a physician before use.
The Social Science team developed a survey tool for hospital health professionals in Europe to assess their perceptions of local infection prevention and control measures and their general wellbeing. Two rounds of surveys covered a total of 2,289 health professionals from 26 European countries. Data analysis was finalized and a manuscript was submitted for publication on 20 November 2020.
Research conducted in WP6 related to the needs and experience of household members informed discussions that fed into updated WHO guidelines on ‘Home care for patients with suspected or confirmed COVID-19 and management of their contacts’.
A recent study shows that scientific advisors found the experience of working during the pandemic rewarding. However, they also faced numerous challenges, including working under significant time constraints to produce ‘evidence’ as quickly as possible. The study high-lights several lessons which can facilitate preparedness for future health emergencies.